Le rôle critique du WMS dans l'entreposage pharmaceutique : Conformité, traçabilité et efficacité
Introduction : La complexité de la chaîne d'approvisionnement pharmaceutique
L'entreposage pharmaceutique ne consiste pas seulement à stocker des produits, mais aussi à préserver des médicaments vitaux, à garantir la sécurité de la santé publique et à respecter des normes de conformité rigoureuses. Le secteur est soumis à une surveillance réglementaire stricte et les risques sont élevés : les médicaments contrefaits, les stocks périmés et les retards, même minimes, peuvent avoir de graves conséquences.

La chaîne d'approvisionnement pharmaceutique est confrontée à de nombreux obstacles réglementaires. Des agences telles que l Administration américaine des denrées alimentaires et des médicaments (FDA) et le Agence européenne des médicaments (EMA) ont établi des lignes directrices complètes pour la fabrication, la distribution et le stockage des produits pharmaceutiques. Les responsables de la chaîne d'approvisionnement doivent comprendre en profondeur ces réglementations et mettre en œuvre des systèmes d'assurance qualité solides pour garantir la conformité. La non-conformité peut avoir des conséquences graves, telles que le rappel de produits, des amendes importantes et des poursuites pénales.
Contrairement à d'autres secteurs, les produits pharmaceutiques - qu'il s'agisse de médicaments sur ordonnance, de médicaments en vente libre ou de produits nutraceutiques - sont très réglementés, très précieux et souvent sensibles à la température et à la manipulation. Les distributeurs et les exploitants d'entrepôts sont soumis à une pression considérable pour maintenir une conformité totale, minimiser les erreurs opérationnelles et assurer des livraisons rapides et précises.
C'est là qu'une Système de gestion des entrepôts (WMS) entre en jeu. Un WMS de qualité pharmaceutique offre des fonctionnalités avancées qui contribuent à garantir la conformité réglementaire, la traçabilité sécurisée et l'efficacité opérationnelle tout au long de la chaîne d'approvisionnement.
Principaux défis en matière d'entreposage de produits pharmaceutiques
1. Respect de réglementations strictes

L'entreposage des produits pharmaceutiques doit se conformer à de multiples cadres réglementaires, notamment
- Directive sur les médicaments falsifiés (FMD) - Règlement de l'Union européenne visant à prévenir la contrefaçon de médicaments.
- Bonnes pratiques de distribution (GDP) - Veiller à ce que les produits soient stockés et transportés de manière cohérente.
- Bonnes pratiques de fabrication (BPF) - S'applique à toutes les étapes de la production et de la logistique pharmaceutiques.
Le non-respect de ces règles peut entraîner la suspension de la licence, des sanctions financières ou des poursuites judiciaires.
2. Risque de médicaments contrefaits
De faux médicaments ou des médicaments falsifiés peuvent entrer dans la chaîne d'approvisionnement sans une sérialisation et une vérification appropriées. Pour s'en prémunir, il faut des données validées, des processus sécurisés et des protocoles logistiques inviolables.
3. Contrôle précis des stocks
De nombreux produits pharmaceutiques nécessitent :
- Durée de conservation courte
- Étiquetage spécifique au lot
- Conditions de stockage particulières
Le maintien de la précision du suivi des lots et des dates de péremption est essentiel pour la sécurité et la conformité réglementaire.
4. Demande de rapidité et de précision
Les patients, les pharmacies et les hôpitaux attendent des livraisons rapides et précises. Les entrepôts pharmaceutiques doivent fonctionner avec une tolérance minimale à l'erreur, que ce soit lors de la préparation des commandes, de l'étiquetage ou de l'expédition.
Fonctions essentielles des systèmes de gestion d'entrepôt pour l'industrie pharmaceutique
1. Gestion de la conformité : Respecter les normes de la fièvre aphteuse
Un WMS spécialisé prend en charge Conformité à la fièvre aphteuse par le biais des éléments suivants :
- Analyse de l'identifiant unique: Interprète les codes-barres GS1 DataMatrix contenant des codes de produits, des numéros de lots, des dates de péremption et des numéros de série.
- Vérification des points de contrôle critiques: Valide les produits lors de la réception, des mouvements internes et de l'expédition.
- Intégration avec NMVS/SecurMed: Interaction en temps réel avec des systèmes nationaux comme celui du Royaume-Uni NMVS via SecurMed pour vérifier l'authenticité du produit.
- Traitement des changements de statut: signale et isole automatiquement les médicaments périmés, rappelés ou volés.
- Gestion des exceptions: soutient les cas de distribution exceptionnels tels que l'utilisation vétérinaire ou les essais cliniques, y compris les exemptions au titre de l'article 23 dans le cadre de la fièvre aphteuse.
- Intégration des systèmes: Connexion transparente avec les systèmes ERP et réglementaires afin de minimiser les saisies manuelles et les erreurs humaines.
2. Sérialisation et traçabilité de bout en bout
- Visibilité des stocks en temps réel: Suivi de chaque mouvement de produit depuis la réception jusqu'à la livraison.
- Gestion des données sérialisées: Associe des identifiants de produits uniques aux activités de fabrication, d'entreposage et de distribution.
- Capacité de piste d'audit: Tient des registres complets et inviolables qui répondent aux attentes de la FDA, de l'EMA et d'autres autorités.
3. Date d'expiration et gestion des lots
- FIFO/FEFO automatisé: Met en œuvre des stratégies "premier entré-premier sorti" ou "premier expiré-premier sorti" afin d'optimiser la rotation des stocks.
- Alertes d'expiration: Avertit le personnel de l'entrepôt des produits dont la date de péremption est proche afin de permettre la prise de mesures proactives.
- Préparation au rappel: Identifie instantanément les lots concernés lors d'un rappel de produit.
4. Contrôle des risques et de la sécurité
- Chaîne du froid Contrôle: Utilise des capteurs IoT pour maintenir la conformité avec les exigences de stockage à température contrôlée.
- Mesures antivol et anti-mélange: Met en œuvre des systèmes de contrôle d'accès, de restriction de zones et d'alarme pour assurer la sécurité.
- Prévention de la contamination: Attribue les produits aux zones désignées et applique les protocoles d'hygiène.
5. Efficacité opérationnelle
- Préparation de commandes et emballage automatisés: Réduit les erreurs humaines et accélère le traitement des commandes.
- Algorithmes de créneaux intelligents: Optimise l'emplacement des produits pour améliorer la vitesse de prélèvement et minimiser la consommation d'énergie.
- Intégration multi-systèmes: Se connecte aux plateformes ERP, logistiques et de commerce en ligne pour une gestion transparente des commandes.
Intégration des technologies et applications dans le monde réel
Intégration de l'IdO
- Les capteurs IoT surveillent en permanence des conditions telles que la température et l'humidité.
- Des alertes en temps réel permettent de prévenir les écarts avant qu'ils ne compromettent l'intégrité du produit.
La blockchain dans la logistique pharmaceutique
- La technologie blockchain garantit l'intégrité et la transparence des données.
- Les enregistrements immuables renforcent la confiance dans les données sérialisées de la chaîne d'approvisionnement, réduisant ainsi les risques de fraude.
Étude de cas : Le WMS permet la conformité à la fièvre aphteuse
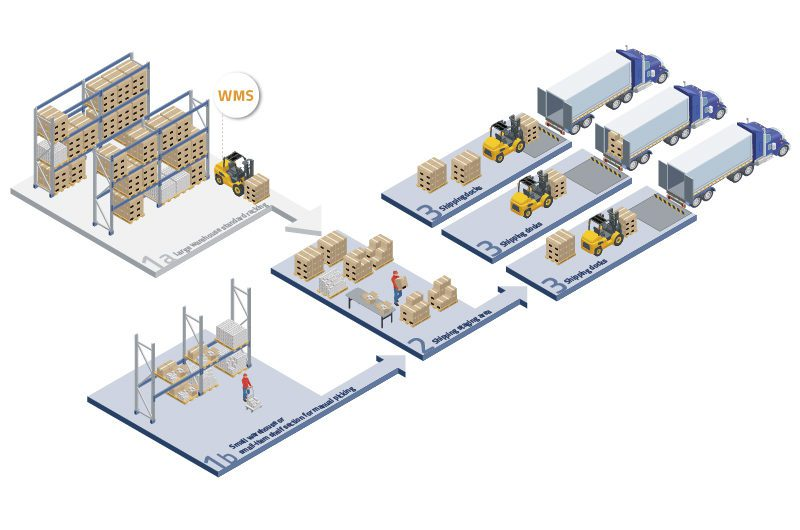
Un important distributeur de produits pharmaceutiques au Royaume-Uni a intégré un WMS au système de vérification national de SecurMed. Les résultats sont les suivants :
- 30% réduction du temps de vérification manuelle des produits
- 100% conformité avec les règles de sérialisation de la fièvre aphteuse
- Amélioration du suivi des lots et de la gestion des produits proches de la date de péremption
Tendances futures en matière d'entreposage de produits pharmaceutiques
1. Logistique pharmaceutique transfrontalière
Avec la mondialisation croissante, les systèmes WMS doivent être compatibles :
- Multiples cadres de conformité dans les différentes régions
- Visibilité des stocks transfrontaliers
- Intégration avec la documentation des douanes et des contrôles frontaliers
2. L'analyse prédictive pilotée par l'IA
L'intelligence artificielle va transformer l'entreposage des produits pharmaceutiques en permettant.. :
- Prévision de la demande: Prévient les ruptures de stock et les scénarios de surstockage.
- Maintenance prédictive: Assurer le temps de fonctionnement des équipements et éviter les défaillances de la chaîne du froid.
- Optimisation dynamique: Ajuste les flux de travail en temps réel en fonction du volume, de la main-d'œuvre et des données d'inventaire.
Conclusion : Une nécessité stratégique pour la logistique pharmaceutique moderne
Dans un secteur où la sécurité, la conformité et la rapidité sont essentielles, un WMS de qualité pharmaceutique n'est plus facultatif, il est indispensable. Qu'il s'agisse de se prémunir contre la contrefaçon de médicaments, d'assurer la traçabilité ou d'optimiser les opérations d'entreposage, les plateformes WMS sont au cœur d'une chaîne d'approvisionnement pharmaceutique résiliente et conforme.
À mesure que l'industrie évolue, ceux qui investissent dans les technologies avancées de l'information et de la communication (TIC) sont de plus en plus nombreux. Technologie WMS seront les mieux placés pour faire face à la complexité de la réglementation, répondre aux attentes croissantes des consommateurs et fournir des produits vitaux de manière fiable et efficace.
Aperçu de l'industrie
nouvelles via la boîte de réception
Nulla turp dis cursus. Integer liberos euismod pretium faucibua