How GS1 Labels Support Global Regulatory Compliance
Governments on every continent continue to sharpen rules that protect consumers, secure supply chains, and guarantee product authenticity. From the U.S. Food & Drug Administration’s Drug Supply Chain Security Act to the EU’s Falsified Medicines Directive, regulations increasingly demand granular product identification, real-time traceability, and data transparency. Companies that ship products across borders often struggle to satisfy these diverse mandates without ballooning costs or fragmenting their information systems.
One proven solution is the adoption of GS1 label standards, which offer a single, interoperable language for product data exchange. By leveraging standardized barcodes, identification keys, and data carriers, organizations can address multiple regulatory requirements with one cohesive strategy.

The Regulatory Landscape: Complex but Converging
Although each region issues unique rules, they share common expectations:
- Unique product identifiers – regulators want to know exactly which item is moving where.
- Serialization and aggregation – many pharmaceutical and tobacco laws mandate serial numbers at unit, case, and pallet levels.
- Event and status reporting – food safety and medical device legislation require timely updates on location, condition, or recall status.
- Data accessibility – customs authorities, market-surveillance agencies, and even end consumers expect fast, reliable access to product data.
GS1 standards directly map to these needs by providing globally unique identifiers (GTIN, SSCC, GLN), standardized barcode symbologies (GS1-128, DataMatrix, QR Code), and structured data formats (EDI, EPCIS).
Core GS1 Components That Support Compliance
1. Global Trade Item Number (GTIN)
Regulators increasingly insist on a single, unambiguous product code. The GTIN fulfills this role, ensuring that the same shampoo bottle or insulin pen is recognized identically in New York, Nairobi, or Nagoya.
2. Serial Shipping Container Code (SSCC)
Many customs and border-protection agencies require advance shipment notices. An SSCC on a GS1 label links every pallet to its electronic shipping manifest, simplifying import clearance.
3. Application Identifiers (AIs)
AIs embed critical data—batch/lot, expiration date, serial number—within the barcode. Different statutes reference different data points, but a well-constructed GS1 barcode can carry them all simultaneously, avoiding label clutter or re-printing.
4. EPCIS and Digital Link
Traceability laws demand event capture (“received,” “packed,” “shipped”) and easy data retrieval. EPCIS documents these events in a standardized way, while GS1 Digital Link turns the GS1 label itself into a URL that can reveal product provenance or recall status to regulators and consumers.
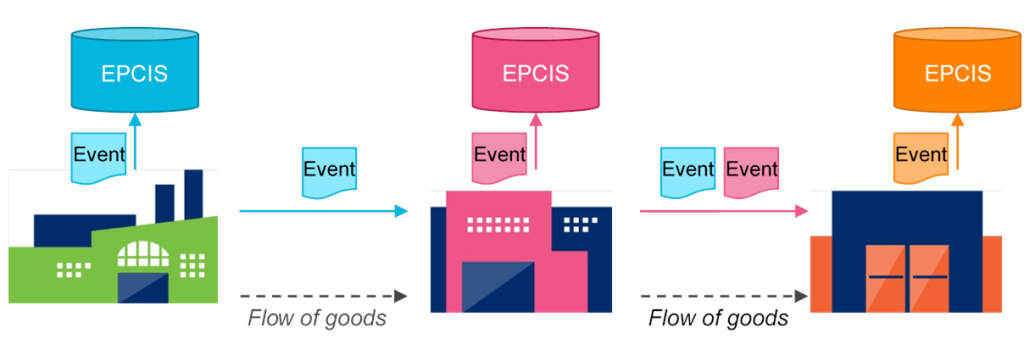
Region-Specific Examples of GS1 Alignment
| Region / Regulation | GS1 Contribution | Speeds withdrawal across the retail chain through standardized codes |
|---|---|---|
| United States – DSCSA | GTIN + serial number in a 2D DataMatrix | Meets interoperable traceability and verification rules for prescription drugs |
| European Union – FMD | GTIN (ECCN), batch, expiry, and unique serial in a 2D code | Enables repository comparison and pharmacy verification |
| China – UDI (Medical Devices) | GTIN as base identifier, AI (17) expiry, AI (10) batch | Harmonizes with international Unique Device Identification expectations |
| Brazil – RDC 423 Food Recall | GTIN and batch in GS1-128 | Speeds withdrawal across retail chain through standardized codes |
| Global – IMO/ISO Maritime Dangerous Goods | SSCC and AI (20) for container numbers | Streamlines customs risk assessment |
In each scenario, the regulator cites a need; the GS1 label supplies the format.
Implementation Best Practices
- Map Data Requirements Early
List every data element demanded by your target markets. Most can be expressed by an existing GS1 Application Identifier. - Select the Right Data Carrier
- GS1-128 suits logistics labels where space permits.
- GS1 DataMatrix or QR codes serve small items requiring high data density.
- Validate with Online Tools
GS1 member organizations offer label-verification portals that test whether your GS1 label meets ISO print quality and AI syntax rules. - Integrate with ERP/WMS
Embed GTINs and SSCCs directly into your master data. Automated printing triggered by ERP events reduces manual keying errors. - Educate Partners and Inspectors
Share decoding guides so distributors, customs officers, and auditors understand the information inside the barcode—accelerating inspections and reducing queries.
Common Pitfalls and How to Avoid Them
- Duplicate Numbers – Re-using a GTIN for a reformulated product can breach both GS1 and regulatory rules. Always assign a new GTIN if ingredients, quantity, or key attributes change.
- Partial Adoption – Printing a GS1 label on cases but not on individual units may fail serialization laws. Ensure hierarchical packaging logic.
- Local Over-Labeling – Adding stickers for each jurisdiction bloats packaging lines. Integrate all required data into one universal barcode where possible.
- Ignoring Verification – Poor print contrast or truncated quiet zones can render codes unreadable, leading to customs holds or retailer refusals. Regular verify-and-grade routines are mandatory.

Measuring Success
Key performance indicators (KPIs) that signal your GS1 program is supporting compliance:
- Scan Pass Rate at Ports and Warehouses – Aim for 99 %+ first-time read.
- Regulatory Inquiry Resolution Time – Faster document retrieval equals fewer fines.
- Recall Execution Speed – Unified GTIN and batch data should enable targeted withdrawals in hours rather than days.
- Label Rework Incidents – Decline over time as master data and printing logic stabilize.
Looking Ahead: Digital Transformation and Beyond
Regulators themselves are embracing digital frameworks. The EU’s forthcoming Digital Product Passport and the FDA’s push for interoperable serialization reporting signal a future where data moves faster than goods. The GS1 label—paired with cloud-based EPCIS and Digital Link endpoints—positions companies to plug directly into these ecosystems without rebuilds.
Conclusion
Global regulations won’t get simpler, but compliance architecture can. By centering your program on the GS1 label and its supporting standards, you create a single source of truth that satisfies customs declarations, product-safety mandates, and consumer transparency all at once. In effect, GS1 becomes the multilingual passport your products carry through every checkpoint, ensuring they travel the world quickly, legally, and confidently.
Industry Insights
news via inbox
Nulla turp dis cursus. Integer liberos euismod pretium faucibua