GS1 Label Requirements for Retail, Pharma, and Food
GS1 labels are vital in today’s global supply chain. These labels contain unique identification codes that provide critical product information, ensuring that items can be tracked, traced, and authenticated at every point in the distribution process. The GS1 standard has become a universal language for identifying and tracking goods across industries, including retail, pharmaceuticals, and food. Understanding the specific GS1 label requirements for these sectors is essential for companies looking to maintain compliance, improve efficiency, and enhance customer satisfaction.
What is a GS1 Label?
A GS1 label includes a barcode or QR code that represents data about a product. This label ensures that every item can be uniquely identified across various stages of the supply chain. The GS1 standard covers several data elements, including product identification, batch numbers, expiration dates, and serial numbers, all encoded in machine-readable formats. This universal system helps businesses streamline inventory management, ensure product safety, and meet regulatory standards.

GS1 Label Requirements for Retail
In the retail industry, the use of GS1 labels is essential for smooth operations, accurate inventory management, and effective communication between suppliers, manufacturers, and retailers. The following are the primary GS1 label requirements for the retail sector:
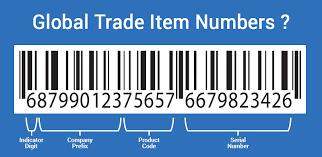
- GTIN (Global Trade Item Number):
The GTIN is a unique identifier assigned to each product. It can be represented in various barcode formats, such as UPC (Universal Product Code) or EAN (European Article Number). For retailers, the GTIN is crucial for accurate inventory tracking, checkout systems, and product categorization.
- Barcode Format:
Retailers primarily use 1D barcodes, typically in EAN-13 or UPC-A formats, to store GTIN data. The barcode must be printed clearly and scannably, ensuring quick and accurate product identification during transactions. - Product Description:
The GS1 label should include a concise description of the product, including its brand, size, and any distinguishing characteristics. This information helps retailers quickly identify products on shelves and streamline the restocking process. - Batch Numbers and Expiration Dates (where applicable):
For products with limited shelf lives or specific manufacturing batches, such as clothing, electronics, or consumer goods, batch numbers and expiration dates must be included on the GS1 label. These ensure that retailers can manage stock rotation efficiently and avoid selling expired products. - Supplier Information:
The label should also include the supplier’s information, allowing for efficient communication and traceability in case of product recalls or inquiries. This is particularly important for managing supply chain risks and ensuring product safety.
GS1 Label Requirements for the Pharmaceutical Industry
The pharmaceutical industry has unique requirements for GS1 labels due to the stringent regulatory frameworks aimed at ensuring drug safety and patient protection. Compliance with these standards is critical for pharmaceutical manufacturers, distributors, and retailers. Here are the key GS1 label requirements for pharmaceuticals:
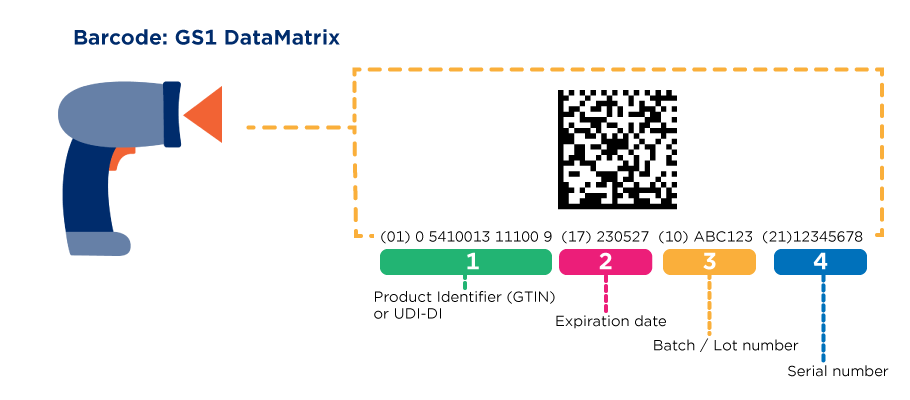
- Serial Numbers and Expiry Dates:
For pharmaceutical products, including prescription drugs and over-the-counter medications, serial numbers and expiration dates are mandatory. These details help with traceability, ensuring that each unit can be tracked from manufacturing through to the point of sale. Serial numbers also play a significant role in preventing counterfeit products from entering the market. - GTIN and Batch/Lot Numbers:
Similar to retail requirements, pharmaceutical products must be assigned a unique GTIN to identify the product. Additionally, each batch or lot of medication is assigned a specific batch number, which allows manufacturers and distributors to trace the product back to its source in case of a recall. - Regulatory Compliance Codes:
Pharmaceutical GS1 labels are often required to include additional regulatory compliance codes such as the National Drug Code (NDC) or specific codes mandated by health authorities in different regions (e.g., FDA in the U.S., EMA in Europe). These codes are essential for maintaining compliance with pharmaceutical laws and regulations. - Two-Dimensional Barcodes:
In the pharmaceutical industry, GS1 labels typically use 2D barcodes, such as the GS1 DataMatrix. This is a more efficient way of encoding a significant amount of information, including serial numbers, expiration dates, and batch numbers. The 2D barcode is used to enhance traceability and improve the efficiency of logistics and inventory management. - Tamper-Evident Features:
To reduce the risk of counterfeiting, pharmaceutical products often require tamper-evident features on GS1 labels. These could include security seals, holograms, or specific markings that allow consumers and regulators to quickly detect if a package has been tampered with.
GS1 Label Requirements for the Food Industry

In the food industry, GS1 labels are crucial for ensuring product safety, managing traceability, and complying with food safety regulations. The following requirements must be met for food products:
- GTIN and Barcode Format:
Similar to other industries, food products must have a unique GTIN for identification. The GTIN is typically represented in an EAN-13 barcode format, allowing retailers and distributors to quickly scan and process products at various stages of the supply chain. - Expiration Date and Best Before Date:
Food products, particularly perishable goods, must display expiration dates or best-before dates clearly on their GS1 labels. This is essential for consumer safety, inventory management, and compliance with food safety regulations. - Batch Numbers and Traceability:
Batch numbers are essential for food traceability. This allows companies to track and manage product recalls efficiently, which is particularly important in the case of contamination or foodborne illness outbreaks. The GS1 label ensures that every product can be traced back to its origin, including the production date and supplier. - Ingredient and Allergen Information:
For food safety reasons, the GS1 label must include ingredient lists, nutritional information, and allergen warnings. This ensures transparency and helps consumers make informed decisions about their purchases. It is also crucial for food manufacturers to meet the labeling requirements set by regulatory bodies such as the FDA or EFSA. - Sustainability Information (Optional):
As consumer interest in sustainability grows, some food products include additional information on their GS1 labels, such as certifications for organic products, fair trade, or environmentally friendly packaging.
Conclusion
The GS1 label plays an indispensable role across various industries, providing essential data to improve product traceability, safety, and compliance. In the retail, pharmaceutical, and food sectors, GS1 labeling requirements ensure that products are properly identified, tracked, and managed throughout the supply chain. Compliance with these requirements not only helps businesses streamline operations and meet regulatory standards but also enhances consumer trust and safety. Whether in retail, pharmaceuticals, or food, using the GS1 system to meet labeling requirements is a step toward a more efficient and secure global supply chain.
Industry Insights
news via inbox
Nulla turp dis cursus. Integer liberos euismod pretium faucibua